
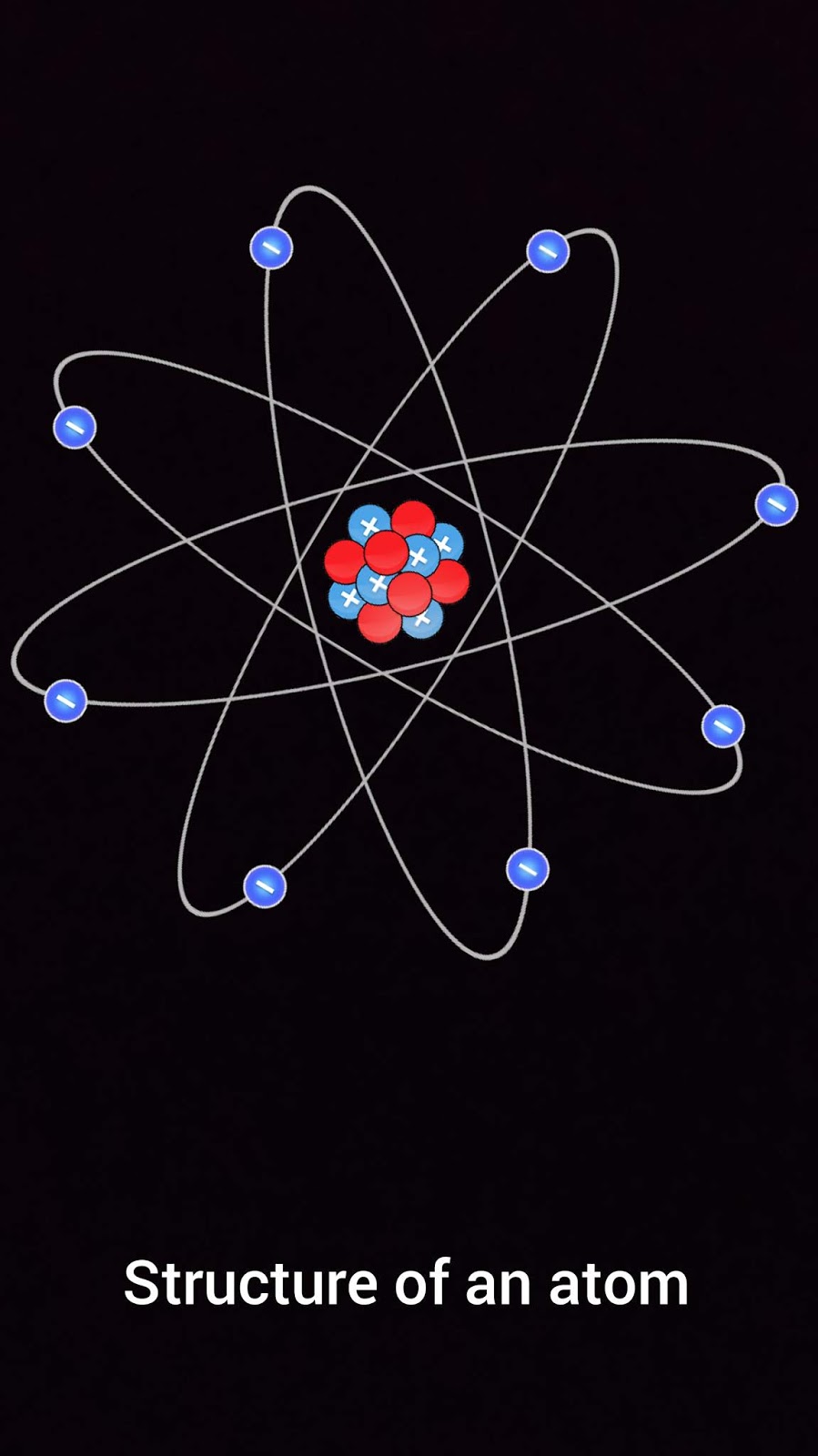
Do ionic compounds tend to have low melting points?
When covalent bonds form between atoms, the resulting entity is called a molecule with a fixed characteristic geometry. When two electrons are shared between two neighbouring atoms, they are said to be joined by a covalent bond. But when sodium and chlorine (Cl) combine, they form a non-reactive substance called sodium chloride (table salt, NaCl). Forces act on the bonds between atoms, changing the molecular structure of a substance. If a molecule forms from atoms of two or more different elements, we call it a compound.Ĭhemical changes in compounds happen when chemical bonds are created or destroyed. For example, when three oxygen atoms bond together, they form a molecule of ozone (O3). Sometimes the atoms are all from the same element. When two or more atoms chemically bond together, they form a molecule. When compounds form when atoms come together? Because they are unstable and can share electrons in their outer energy levels. Why do most elements tend to form compounds. What happens to the properties of elements when atoms form compounds? They share electrons and form a “compound bond”. What happens to atoms when they form compounds? 6 What happens in a double replacement chemical reaction?.5 How are covalent bonds formed in a compound?.4 Do ionic compounds tend to have low melting points?.2 When compounds form when atoms come together?.1 What happens to atoms when they form compounds?.


 0 kommentar(er)
0 kommentar(er)
